X-Ray Fluorescence spectrometer (XRF)
|

XRF Laboratory |
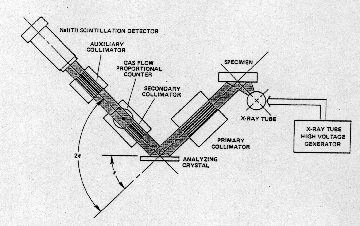
| X-Ray Fluorescence Sepectrometry (WDXRF) (Wavelength - despensive x-ray flourescence spectrometry is one of the most widely used
and versatile of all instrumental analytical techniques. An XRF
spectrometer uses primary radiation from an X-ray tube to excite
secondary emission from a sample. |
| The radiation emerging from the
sample includes the characteristic X-ray peaks of major and trace
elements present in the sample. Dispersion of these secondary X-rays
into a spectrum, usually by X-ray diffraction, allows identification of
the elements present. The height of each characteristic X-ray peak
relates to the concentration of the corresponding element in the
sample, allowing quantitative analysis of samples for most elements in
the concentration range 1 ppm to 100 %.
To avoid particle size effects, rock samples should be ground as finely
as possible, but the only way to eliminate particle size effects
completely is to homogenise the sample by fusion. A mixture of
lithiummetaborate and lithiumtetraborate is used for this purpose and
fused with the sample in the ratio 1 part sample to 5 parts flux. Fused
glass beads are only used in the determination of the major elements
where the particle size effects are most dominant. Trace elements are
determined on pressed powder samples made by mixing the powdered rock
with a binder and pressing a pellet at high pressure. Operation is fully automatic and results are typically delivered within hours, minutes or even seconds. |
|

